Key discoveries by our group include:
Pharmacologic inhibition of Plk1 elicits graded effects
- Lera RF, Burkard ME. High mitotic activity of Polo-like Kinase 1 is required for chromosome segregation and genomic integrity in human epithelial cells. J Biol Chem, 2012 Dec 14;287(51):42812-25. PMCID: PMC3525009.
Human cells that fail cytokinesis can evade genome doubling through cytofission
- Choudhary A, Lera RF, Martowicz ML, Oxendine K, Laffin J, Weaver BA, Burkard ME. Interphase cytofission maintains genomic integrity of human cells after failed cytokinesis. Proc Natl Acad Sci USA, 2013 Aug 6; 110(32):13026-31. PMCID: PMC3740861.
Plk1 exhibits spatially distinct functions and catalysis in the kinetochore
- Lera RF, Potts GK, Suzuki A, Johnson JM, Salmon ED, Coon JJ, Burkard ME. Decoding Polo-like kinase 1 signaling along the kinetochore-centromere axis. Nature Chem. Biol. 2016 doi:10.1038/nchembio.2060. PMCID: PMC4871769.
Development of new methods to standardize measures of Chromosomal instability
- Lynch AR, Arp NL, Zhou AS, Weaver BA, Burkard ME. Quantifying chromosomal instability from intratumoral karyotype diversity using agent-based modeling and Bayesian inference. Elife, 11:e69799, 2022. PMCID: PMC9054132
- Lynch AR, Bradford S, Zhou AS, Oxendine K, Henderson L, Horner VL, Weaver BA, Burkard ME. A survey of CIN measures across mechanistic models. Proc Natl Acad Sci USA., 121 (16)e2309621121 (Preprint: bioRxiv 2023.06.15.544840). PMCID: PMC10312700
Key discoveries with collaborators:
- Taxol operates by increasing chromosomal errors in dividing cells, not mitotic arrest. (Scribano et al. Sci Transl Med 2021: eabd4811.
- Isometric 12x expansion microscopy can illuminate the kinetochore. (Norman et al. JCB 2025 224:e202407116)
Using chemical genetics to understand how protein kinases control cell division
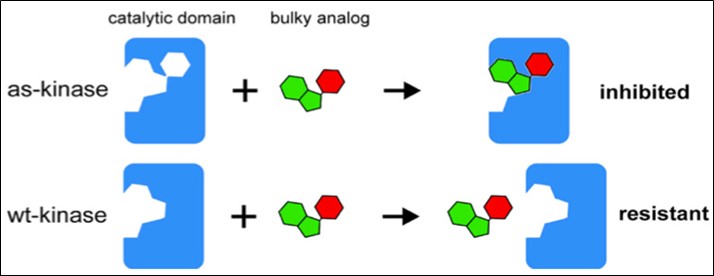
Source: Lera & Burkard, Molecules, 2012
Polo-like kinase 1 (Plk1) is a key mitotic regulator and cancer therapy target, but its broad phosphorylation activity complicates functional analysis. We developed a chemical-genetic toolkit with analog-sensitive Plk1 variants to dissect its roles with spatial and temporal precision.
In collaboration with Prof. Joshua Coon’s lab, phosphoproteomic profiling revealed that Plk1 functions in distinct subcellular pools at the inner kinetochore and during cytokinesis. Mapping these phosphorylation events may uncover new avenues for therapeutic targeting.
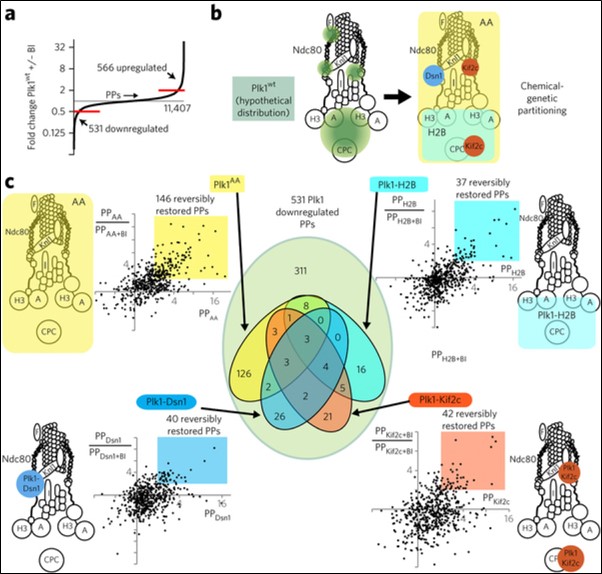
Source: Lera et al, Nat Chem Bio, 2016
Precision Medicine
Identifying Vulnerabilities of Cancer
Polyploidy/Aneuploidy
Polyploidy is common in cancer and linked to poor prognosis. We identified DBPQ and 8-azaguanine as compounds with polyploid-selective activity.
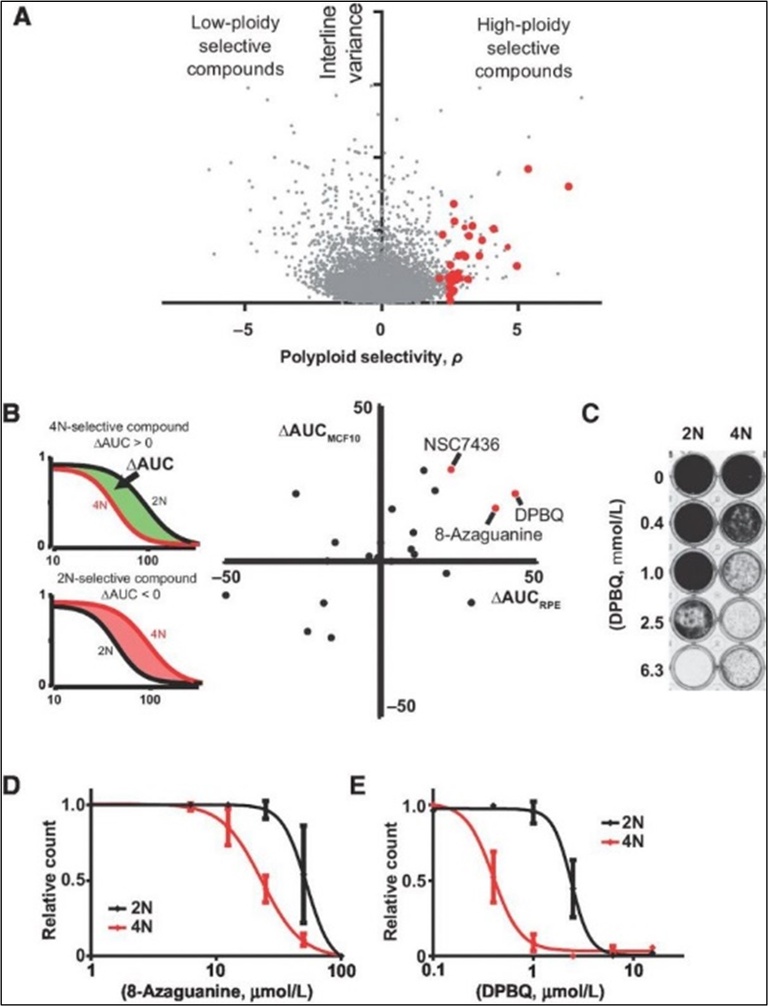
Source: Choudhary et al, Mol Cancer Ther, 2016
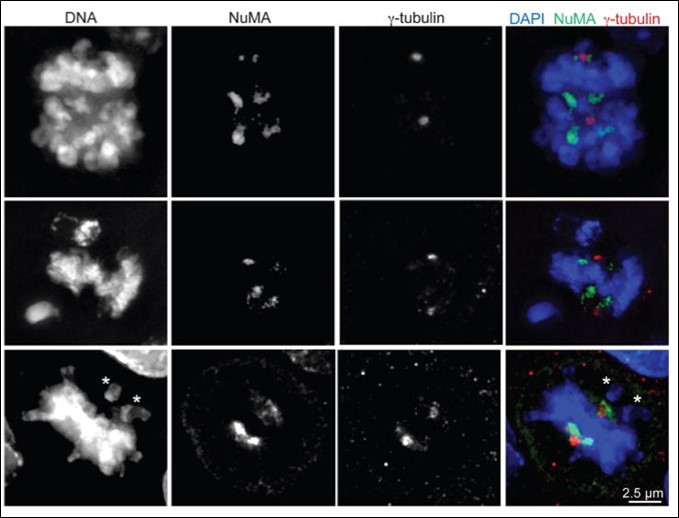
Centrosome Amplification
Centrosome amplification, frequent in cancer and associated with poor prognosis, promotes error-prone mitosis. We study its causes and effects to uncover novel targetable pathways for therapy.
Source: Researchers probe cell division defects to gain insight into cancer
Identifying biomarkers to better target our current therapeutics
Chemotherapy response varies among breast cancer patients but predicting who will benefit remains a challenge. Our lab, in collaboration with Dr. Beth Weaver’s group, investigates biomarkers of paclitaxel response. Contrary to prior belief, we've shown that mitotic arrest is not essential for its effectiveness—suggesting paclitaxel may act by inducing multipolar spindles.